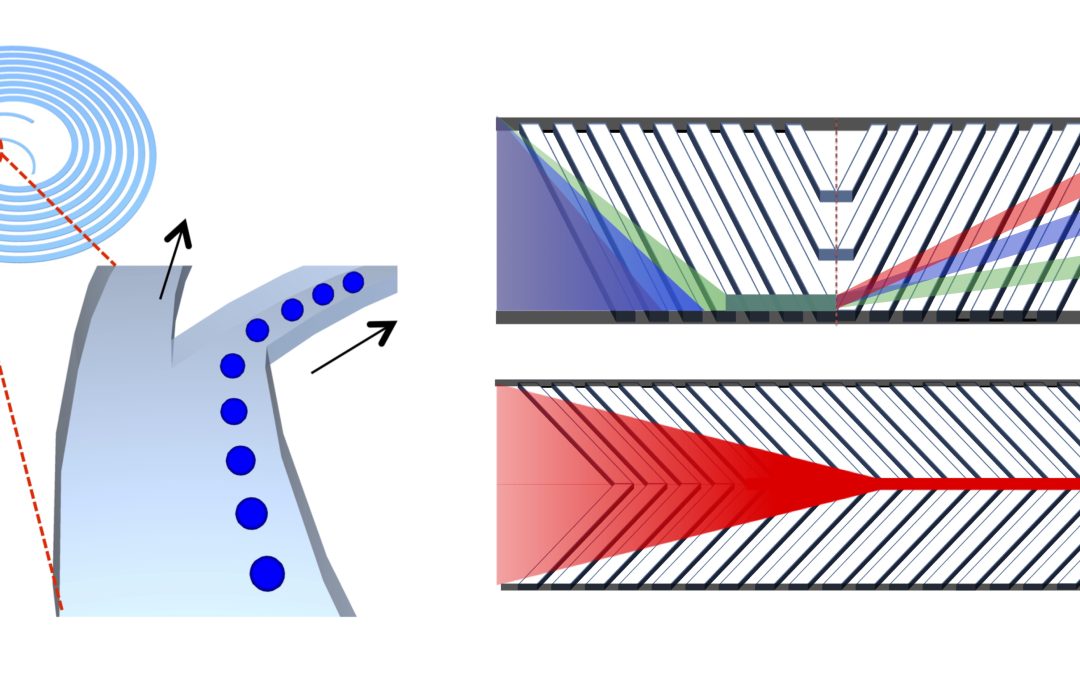
High-quality complex biopharmaceutical products based on cells and proteins are transforming modern medicine and advancing treatments for many health conditions. Continuous biomanufacturing is one of the top technology trends in the biopharmaceutical industry to improve biological product quality and reduce manufacturing cost. Our research introduces novel high-throughput microfluidic cell separation [1, 2, 4, 5, 6] and nanofluidic protein quality monitoring technologies [3, 4]. This novel micro/nanofluidic system [4] enables reliable and efficient microfiltration and robust online rapid product quality monitoring during continuous biomanufacturing. The technology overcomes the limitations of the current membrane-based microfiltration and quality monitoring technologies, including filter clogging, low product recovery, manual sample preparation, and off-line analysis.
First, we developed the novel cell retention device for perfusion culture based on inertial sorting [1, 5, 6]. Size-dependent hydrodynamic forces enabled membrane-less microfiltration for the separation of suspended mammalian cells. The device performance in terms of cell retention efficiency, long-term biocompatibility, and scalability was assessed. Long-term and small-scale perfusion culture using the device was subsequently demonstrated. Clog-free cell retention with high product recovery in this work can be utilized for long-term reliable and efficient perfusion culture.
Next, we introduced the removal of small dead cells from bioreactor cultivation by high-throughput size-based cell separation using inertial sorting [2]. The device parameters were studied to optimize the removal of the dead cells, and high-throughput and high-concentration dead cell removal was demonstrated.
Finally, continuous online purity monitoring of the proteins in the cell culture supernatant during perfusion culture was achieved with a novel nanofluidic filter array [3, 4]. This nanofluidic device with online sample preparation system was integrated with perfusion culture using the microfluidic cell retention device. The purity of proteins in the cell culture supernatant was monitored for more than a week in a fully automated continuous manner. As a robust online sensing technology for continuous biomanufacturing, this nanofluidic filter array could replace the existing offline analytical technologies for protein purity monitoring.
In summary, our work presents a novel micro/nanofluidic system for separation and monitoring of cells and proteins for continuous biomanufacturing [4]. This innovative approach can contribute to long-term reliable and efficient biomanufacturing in the future.
References:
[1] Kwon, T., Prentice, H., Oliveira, J. De, Madziva, N., Warkiani, M. E., Hamel, J.-F. P., & Han, J. (2017). Microfluidic Cell Retention Device for Perfusion of Mammalian Suspension Culture. Scientific Reports, 7, 6703. https://doi.org/10.1038/s41598-017-06949-8
[2] Kwon, T., Yao, R., Hamel, J. F. P., & Han, J. (2018). Continuous removal of small nonviable suspended mammalian cells and debris from bioreactors using inertial microfluidics. Lab on a Chip, 18(18), 2826–2837. https://doi.org/10.1039/c8lc00250a
[3] Ko, S. H., Chandra, D., Ouyang, W., Kwon, T., Karande, P., & Han, J. (2017). Nanofluidic device for continuous multiparameter quality assurance of biologics. Nature Nanotechnology, 12(8), 804–812. https://doi.org/10.1038/NNANO.2017.74
[4] Kwon, T., Ko, S. H., Hamel, J.-F. P., & Han, J. (2020). Continuous online protein quality monitoring during perfusion culture production using an integrated micro/nanofluidic system. Analytical Chemistry, 92(7), 5267-5275, https://doi.org/10.1021/acs.analchem.9b05835
[5] Yin, L., Au, W. Y., Yu, C. C., Kwon, T., Lai, Z., Shang, M., Warkiani, M. E., Rosche, R., Lim, C. T., & Han, J. (2021). Miniature auto-perfusion bioreactor system with spiral microfluidic cell retention device. Biotechnology and Bioengineering, https://doi.org/10.1002/bit.27709
[6] Kwon, T., Choi, K., & Han. J. (2021). Separation of Ultra-high-density Cell Suspension via Elasto-inertial Microfluidics. Small, https://doi.org/10.1002/smll.202101880
Author: Taehong Kwon