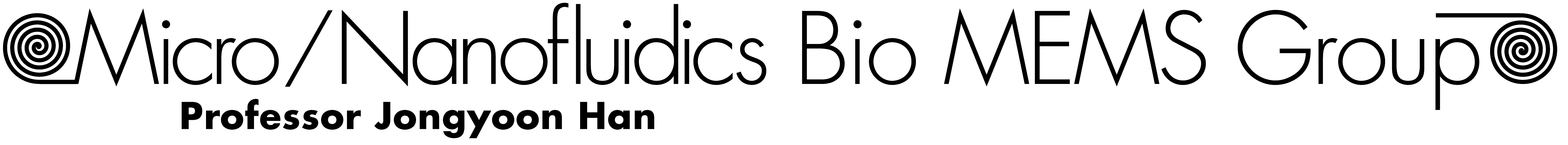Neurological diseases, such as chronic pain, have long resisted chemical intervention strategies. Therapies based on electrical stimulation have emerged to confront the limitations of current treatments, but neural prosthetic devices still face obstacles in terms of specificity and efficacy.
In our work, we take advantage of the fact that neural tissue is highly sensitive to changes in the extracellular concentration of ions such as Ca2+, Mg2+, K+, and Na+. Our core technology is the ion-selective membrane (ISM), which we employ as an electrode coating. This membrane is essentially a filter that we can make selective for virtually any ion. By driving electric current across the electrode and ISM, we can modulate the concentrations of those selected ions in a localized volume around the electrode. Employing devices based on ISM-coated electrodes, we aim to affect our operating principle as a means of pulling different levers in the nervous system than we could with traditional electrical stimulation.
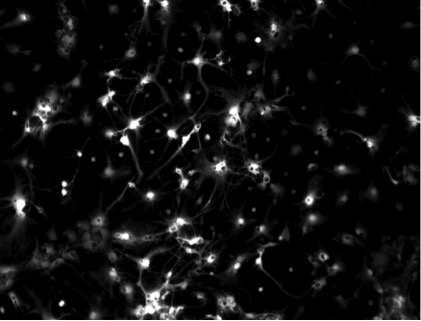
Our current efforts focus on treatment of chronic pain and epilepsy, with targets in the peripheral and central nervous systems respectively. At this stage, we are evaluating the performance of our device in a rat sciatic nerve (acute in vivo) model and a rat hippocampal neuron culture model (see Figure 1).
Figure 1—Ca2+-fluorescence image of dissociated culture of rat hippocampal neurons.
Ref:
1. M. T. Flavin, D. K. Freeman, J. Han, “Interfacial ion transfer and current limiting in neutral-carrier ion-selective membranes: A detailed numerical model,” Journal of Membrane Science, 572, 374-381 (2019)